Research & Development
AMW is characterized by an innovative research and development strategy based on cutting-edge technology and know-how. From preclinical development to upscaling to GMP clinical manufacturing, each development step is carefully planned to develop superior drug delivery solutions. The patient is the focus when advanced therapies are created by combining unique technology with pharmaceutical and biotechnological expertise.
Numerous therapeutic approaches, spanning across a variety of clinical areas, present a significant challenge for both patients and healthcare professionals. From complex administration protocols to dosage control and the effectiveness of treatments, there are substantial gaps in patient care that need to be addressed. Excelling in meeting these unmet clinical needs, controlled-release biodegradable implants emerge as a game-changer, offering several key benefits:
- Maintenance of a steady and prolonged concentration of the active pharmaceutical ingredient (API)
- A one-time injectable solution, reducing the need for repeated administrations by releasing the API over several weeks or months
- Efficient solution for achieving high therapy adherence
- Potential application in areas difficult to reach by the systemic blood circulation
- Prevention of unintended or intended overdosage
The AMW Way
At AMW, the development process thrives on unmatched expertise and a rich history of experience, encompassing everything from the early stages of formulation development to clinical and commercial manufacturing. With a broad range of competencies, AMW functions as a ‘single source’ entity, embarking with an optimal formulation design grounded in biodegradable polymeric matrices. Chemical components, with a primary emphasis on polymers, are carefully chosen and prototyped to synergize with the API integration process, where it may be dispersed or partially dissolved within the matrix to achieve optimal therapeutic efficacy. Subsequently, the drug matrix undergoes meticulous engineering through a progression of technical phases, each centered on various optimizations, including:
- Limiting the molecular mobility of the API to achieve controlled release
- Conducting miscibility tests to address solubility challenges
- Robust extrusion process with reproducible results that minimizes thermal stress on polymer and active ingredient
- Undertaking solid-state characterization of the implant
- Implementing state-of-the-art chemical analytics
In the wake of the galenic development, AMW employs streamlined processes of the highest standards to transition to an in-house, quality-certified GMP manufacturing facility, primed for both clinical and subsequent commercial batch production.
Quality by Design
Research & Development stands as a cornerstone at AMW, reflecting the organization’s intrinsic commitment to innovation. Upheld by high standards and bolstered by state-of-the-art in-house laboratories, our working methods diligently adhere to the Quality by Design (QbD) approach.
Operational procedures are meticulously crafted to satisfy international benchmarks of excellence during the initiation of new projects and the development of novel drug formulations. Our adept R&D team orchestrates the analytical resources and protocols, integrating them seamlessly through the following Process Analytical Technology (PAT) approaches:
Material characterization and formulation
- Microscopic Image Analysis: This technique is crucial for assessing the morphological characteristics of the formulation. It helps understanding the structural properties at a microscopic level, including determining the particle size distribution of the API in solid dispersions, which is vital for optimizing the formulation and predicting its release kinetic.
- ATR-FTIR Spectroscopy: Employed for the chemical characterization of materials. It provides insights into the molecular components and their interactions, playing a key role in ensuring the stability and compatibility of different components within the formulation.
- GPC (Gel Permeation Chromatography): This method determines the molecular weight distribution of polymers in the formulation, a critical aspect in predicting the behavior and performance of the product.
- DSC (Differential Scanning Calorimetry): Utilized to investigate the thermal properties of the materials, offering critical data on glass transitions, melting points, crystallization, and other thermal behaviors, facilitating the optimization of the manufacturing process.
- Rheology: This technique measures the flow properties of formulations, which helps in understanding how the materials will behave during the manufacturing process, ensuring smooth processing and high product quality.
Analytical testing
- HPLC/UPLC with UV/RI/Fluorescence & MS Detection: These are techniques used for the quantitative analysis of various components in the formulation, providing detailed insights into the composition and purity of the product.
- HS-GC (HeadSpace Gas Chromatography): Employed for the analysis of volatile compounds in the formulation, Although GC is only used in special cases when analyzing biodegradable implants compared to GPC and HPLC, it remains an important tool to ensure product stability and safety in specific scenarios.
- Coulometric Water Content Determination: A method used to determine the water content in the formulation, which can influence the stability during the extrusion process as well as the degradation und release kinetic.
Product testing and validation
- Mechanical Material Testing: Utilized to assess the mechanical properties of the product, helping in understanding how the product will behave under various physical conditions.
- In-vitro-Release Tests According to Ph. Eur., USP Own Methods: These tests are conducted to determine the release profile of the active ingredients, offering insights into the efficacy of the product. If necessary, however, the tests can be modified to take account of the special properties of biodegradable polymers, which is central to the evaluation of potential clinical product efficacy.
- ICH Stability Studies (all climate ranges): Conducted to assess the stability of the product under various climatic conditions, providing critical data on the shelf-life and safety of the product.
The extensive array of analytical technologies we utilize enables continuous and personalized method development, along with subsequent validation tailored to meet the specific needs of each individual project. With licenses to handle controlled substances, we enable the implementation of new RD initiatives, supported by extensive resources and strict adherence to regulatory standards. Our commitment is focused on driving innovation, whether through our own developments or by promoting collaborative partnerships.
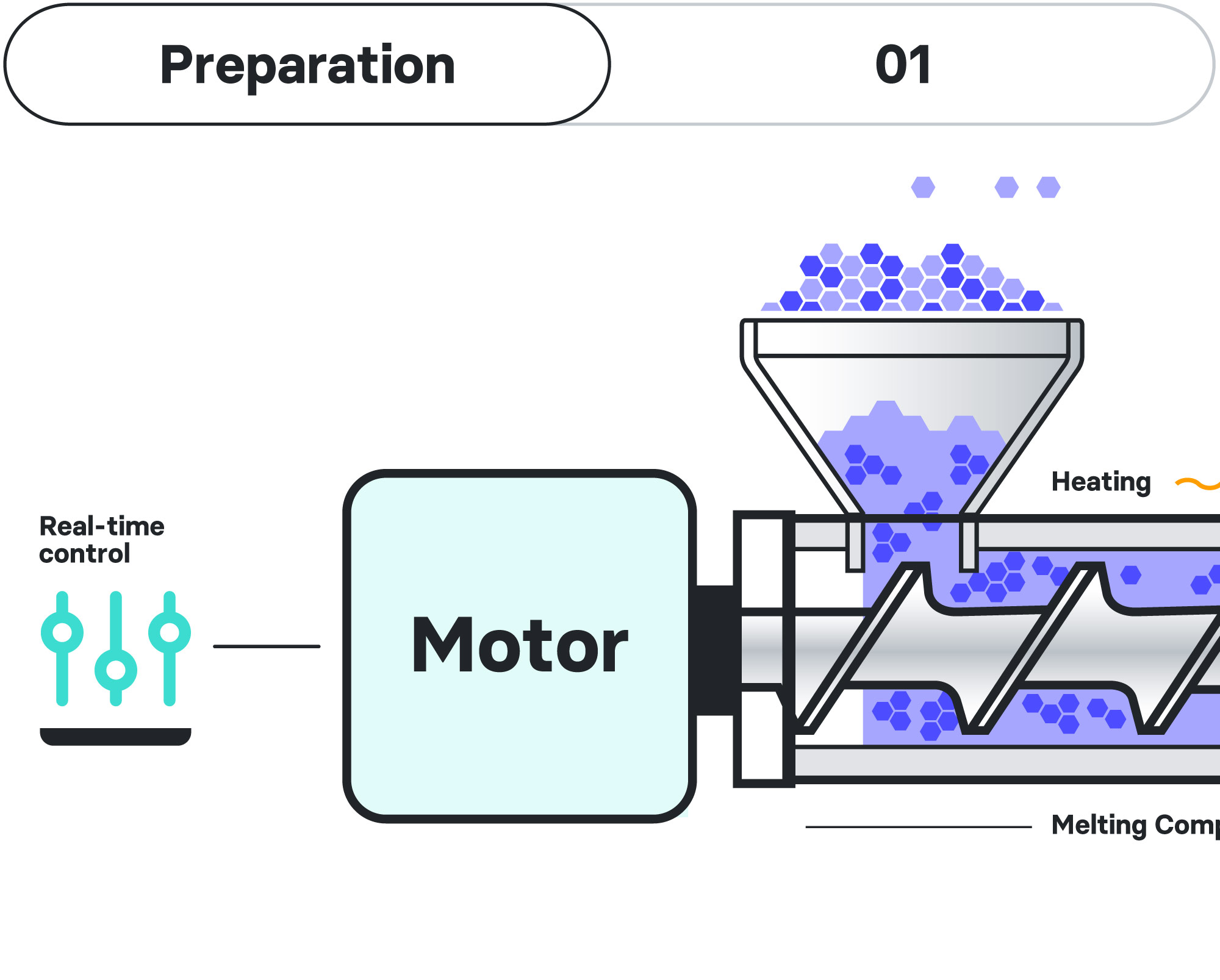
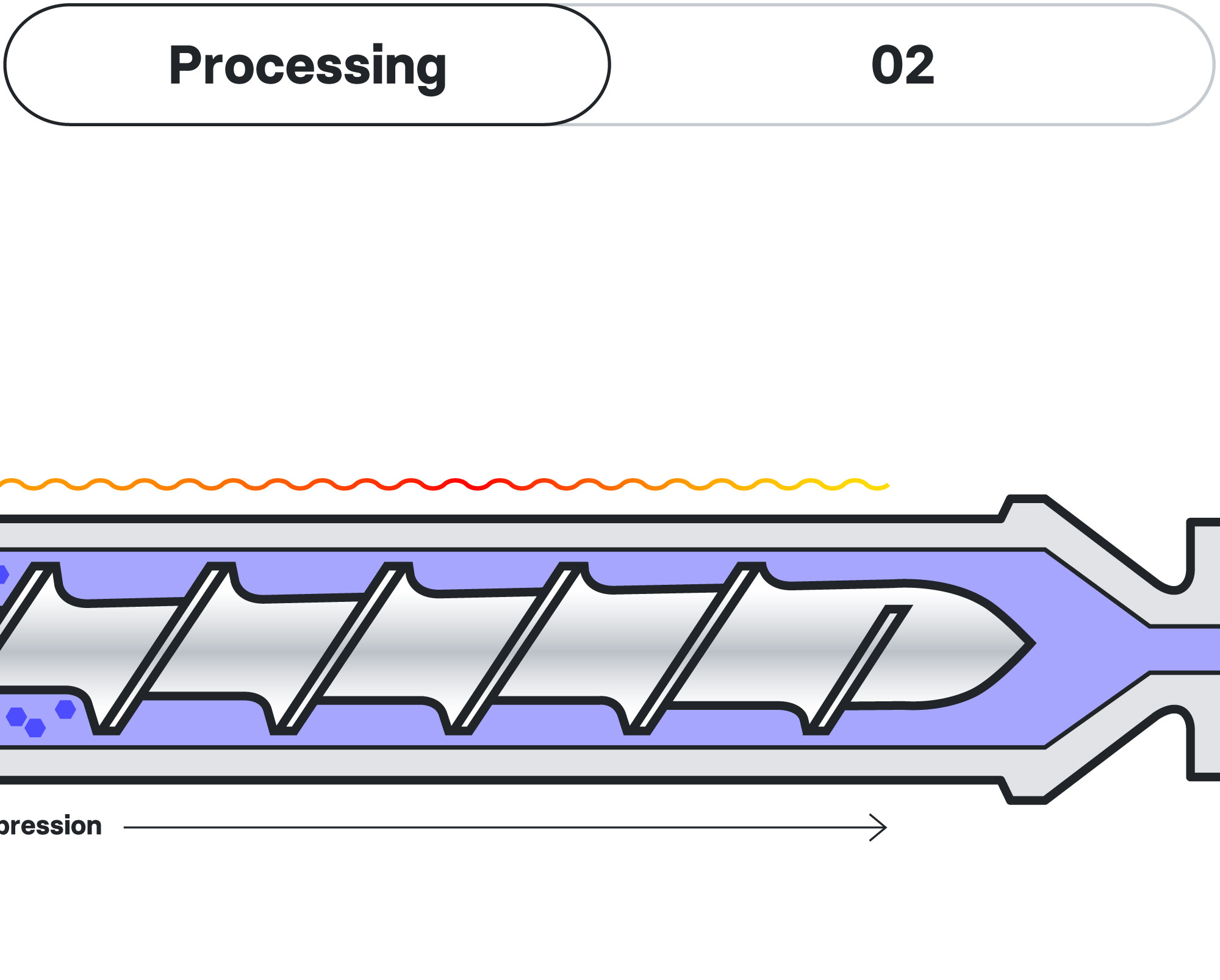
Hot Melt Extrusion (HME)
AMW’s biodegradable implants are crafted using the sophisticated Hot Melt Extrusion (HME) platform technology, a significant player in the realms of pharmaceutical formulation, product development, and manufacturing. This method has been finely tuned to facilitate top-quality, long-acting drug delivery. Within a controlled environment, a blend of polymers merges with a specific API, inducing a transformation in its physical properties and culminating in a solid dispersion of the molecule within the newly formulated compound. The melt is formed under pressure into the desired shape, usually rod-shaped, and cut into the desired length.
This procedure enhances the solubility of the active ingredient, which would otherwise demonstrate limited water solubility in its crystalline state. To ensure a gradual and controlled release, the implants are designed with custom-tailored properties, both in composition and structure. This is achieved through meticulous optimization of its polymeric matrix, a strategy that guarantees predictable and reproducible hydrolysis, along with optimized drug bioavailability.
Hot Melt Extrusion (HME) has emerged as a popular technique in pharmaceutical development, especially in creating sustained-release formulations for local drug delivery.
Advantages HME holds over other formulation techniques
- Enhanced Solubility and Bioavailability: HME can enhance the solubility of poorly water-soluble drugs, which can consequently improve their bioavailability in the body.
- Continuous manufacturing: HME is well-suited for continuous manufacturing, which can potentially lead to reduced manufacturing costs, more consistent product quality, and easier scale-up from research to commercial production.
- Fewer Solvents: Unlike other techniques, HME often requires fewer or no solvents, which can simplify the formulation process and make the technique more environmentally friendly.
- Flexibility in Formulation: HME allows for the incorporation of various drug loads and can be used to create a wide range of dosage forms, including tablets, capsules, and films, offering flexibility in formulation development.
- Precise control of the release rate: The technique allows for precise control over the release rates of active ingredients. This is achieved through careful selection and manipulation of the polymers and processing conditions used during the extrusion process.
- Uniform Dispersion of API: HME can produce formulations with a uniform dispersion of the active pharmaceutical ingredient (API), which can lead to more consistent dosing and potentially reduced side effects.
- Complex Matrix Formulation: It enables the development of complex matrix formulations, which can house multiple active ingredients with varying release profiles, a feature that is beneficial in developing combination drug therapies.
- Stability: Formulations created through HME often exhibit high stability, protecting sensitive APIs from degradation during the shelf-life of the product.
- Regulatory Acceptance: HME has gained acceptance from regulatory agencies for the development of various drug products, which can potentially ease the regulatory approval process.
Limitations
While Hot Melt Extrusion (HME) has become an integral technique in pharmaceutical development, it is not devoid of limitations, especially concerning the selection and processing of Active Pharmaceutical Ingredients (APIs). Here are some of the potential limitations and requirements for APIs in HME:
- Thermal stability: APIs sensitive to heat might undergo degradation during the extrusion process, which generally involves high temperatures. This necessitates the selection of APIs that are thermally stable.
- Complexity in Formulation: Developing formulations with precise drug release profiles can sometimes be complex and requires extensive research and development to optimize the properties of the formulation.
- Physical and Chemical Interactions: There can be physical and chemical interactions between the API and the polymers used in the extrusion process, potentially affecting the stability or release properties of the final product.
- Cost factors: The investment costs for an extrusion line can be considerable, especially when you consider the highly specialized machinery and the necessary analytical instruments. Furthermore, the costs can escalate if specialized polymers are used in the formulation.
- Limited to Certain APIs: Not all APIs may be suitable for HME due to their physical and chemical properties. Finding compatible polymers for certain APIs can be challenging.
API requirements
- Thermal Stability: The API should possess good thermal stability to withstand the high temperatures usually encountered during the extrusion process without undergoing degradation.
- Compatibility with Polymers: The API should be compatible with the polymers and other excipients used in the formulation. It should maintain its stability and efficacy throughout the manufacturing process.
- Adequate Solubility: APIs used in HME should have adequate dispersability in the polymeric matrix to ensure uniform distribution and effective release from the formulation.
- Particle Size: The particle size of the API may need to be carefully controlled to achieve the desired drug release profile and to ensure uniform dispersion in the polymeric matrix. The optimum particle size is determined in preliminary tests and should vary only to a controllable and reproducible extent throughout the process chain.
- Crystallinity: The crystallinity and crystalline form of the API can influence its release properties. In some cases, APIs may need to be processed to modify their crystallinity before being used in HME.
With nearly two decades of expertise in dealing with these challenges and requirements, AMW has advanced know-how in using melt extrusion to develop and manufacture pharmaceutical products with optimized properties.
Versatile innovation & cooperation opportunities
At AMW, the journey of innovation is defined by broad-ranging development avenues and dynamic working models. Our core technology is versatile, encompassing a diverse spectrum of clinical areas and integrating various chemical entities as active therapeutic components. This landscape nurtures a robust pipeline, paving the way for abundant opportunities for collaborations and partnerships with third-party pharmaceutical, biotechnology, and medtech entities under various flexible arrangements.
In-house innovation
Leveraging identified and profiled unmet clinical needs in the sphere of local drug delivery, the AMW teams are dedicated to the proprietary development of novel drug delivery solutions. These initiatives are designed to offer superior treatment alternatives characterized by controlled-release mechanisms. By examining various indications, we strategically reformulate high-potential molecules and create new application devices, aiming to revolutionize healthcare delivery.
Collaborative development
AMW acts as a reliable “single source” partner for the pharmaceutical development of new API drug delivery formulations. Our business models encompass the initiation and nurturing of co-development projects, ranging from foundational RD to streamlined clinical and commercial manufacturing. To ensure the seamless progression of each project, we foster a collaborative workstream, meticulously orchestrated for sustained project management, offering comprehensive solutions and resources at every stage of development.
Customized manufacturing solutions (CDMO)
For partners seeking unique expertise and world-class production capabilities for biodegradable implant formulations, AMW provides customized manufacturing services. After facilitating a smooth process transfer, we guarantee a comprehensive and certified scale-up, anchored in GMP manufacturing units paired with stringent quality control operations, thereby ensuring product excellence at every stage.
Sustainable pipeline
mRNA eluting biodegradable implant as a next-generation gene therapy pathway. Prototyping and plausibility studies concluded.
Goserelin ready-to-use applicator in approval process for Latin America
Biodegradable implant for intravitreal use releasing the active ingredient over a period of 6 months. It is used for the treatment of age-related macular degeneration (AMD), diabetic retinopathy, and retinal vein occlusion.
Development of a biodegradable implant for the delivery of GLP-1 agonists, indicated for the treatment of metabolic syndrome. For the active ingredient, promising results from feasibility and human pharmacokinetic studies are available.
Development of a biodegradable implant for the delivery of GLP-1 agonists, indicated for the treatment of metabolic syndrome. The feasibility study is expected shortly.
n.d.