
Products
AMW develops and manufactures value-added medicines to optimize therapeutic outcomes and enhance patient well-being. A drug consists of two parts, an active ingredient or a mixture of active ingredients and a dosage form (drug delivery system), which plays a decisive role in the efficacy of a drug.
Our controlled-release formulations release the active ingredients over a long period of time. In terms of efficacy and tolerability, there are certain advantages of these formulations compared to conventional dosage forms. In addition, costs for therapies are reduced due to higher efficacy.
The drug delivery systems developed at AMW are parenteral sustained release drug delivery systems. This means that the active ingredients enter the body “bypassing the intestine” and remain chemically unchanged by avoiding the gastrointestinal tract. This results in reduced stress on the body, as active ingredients are not degraded by central organs, such as stomach, liver, kidney and intestines.
AMW’s drug delivery systems can contain various active ingredients. These can be generic, already approved or completely novel active ingredients. Hormones, monoclonal antibodies and nucleic acids are also suitable for application with AMW technology.
Implants goserelin and leuprorelin
AMW has two approved products, goserelin and leuprorelin implants, which are distributed worldwide through both local and global pharma partners.
Both implants contain active ingredients that belong to the class of GnRH agonists (analogues of gonadotropin releasing hormone) which interfere with the regulation of the sex hormone balance. As a consequence, testosterone levels are lowered in men and estradiol levels are lowered in women. This artificially induced lowering of hormone levels enables the symptomatic therapy of hormone-dependent tumors such as prostate cancer or breast cancer.
Indication prostate cancer
Prostate cancer cannot be cured as roughly one-fifth of all tumor cells are hormone resistant. However, 80 percent of patients achieve remission, which means a (temporary) decrease in chronic symptoms. Goserelin and leuprorelin each are an analog of natural gonadoliberin, which is released in a pulsatile manner and binds to receptors in the anterior pituitary, a part of the brain. Both agents cause the release of luteinizing hormone (LH) in the anterior pituitary. LH is required for the production of testosterone in males.
If the pituitary gland is not acting pulsating but continuously, the anterior pituitary lobe becomes insensitive to the excitation (“down-regulation”). Thus, the sustained action of goserelin – after an initial stimulation – causes a sharp decrease in pituitary hormone levels and, as a result, an inhibition of testosterone production.
In principle, these implants achieve the same testosterone level as after removal of one or both testicles (orchiectomy). Clinical studies have demonstrated nearly equivalent therapeutic success by using implants, thereby avoiding the trauma of orchiectomy. In addition, implant therapy shows less undesirable side effects than treatments with other medications, such as diethylstilbestrol.
Goserelin
The active ingredient goserelin is a synthetic peptide that can be used primarily in the treatment of hormone-dependent tumors, particularly prostate cancer. Thus, goserelin intake has indirect effects on testosterone levels in men, the lowering of which stops the growth of malignant cancerous tumors. In addition, goserelin implant is used to treat certain benign gynecological disorders. Goserelin is administered in the form of a biodegradable implant that releases the active ingredient over a period of either one (3.6 mg dosage) or three (10.8 mg dosage) months. Goserelin is the only generic of the originator with global availability.
Leuprorelin
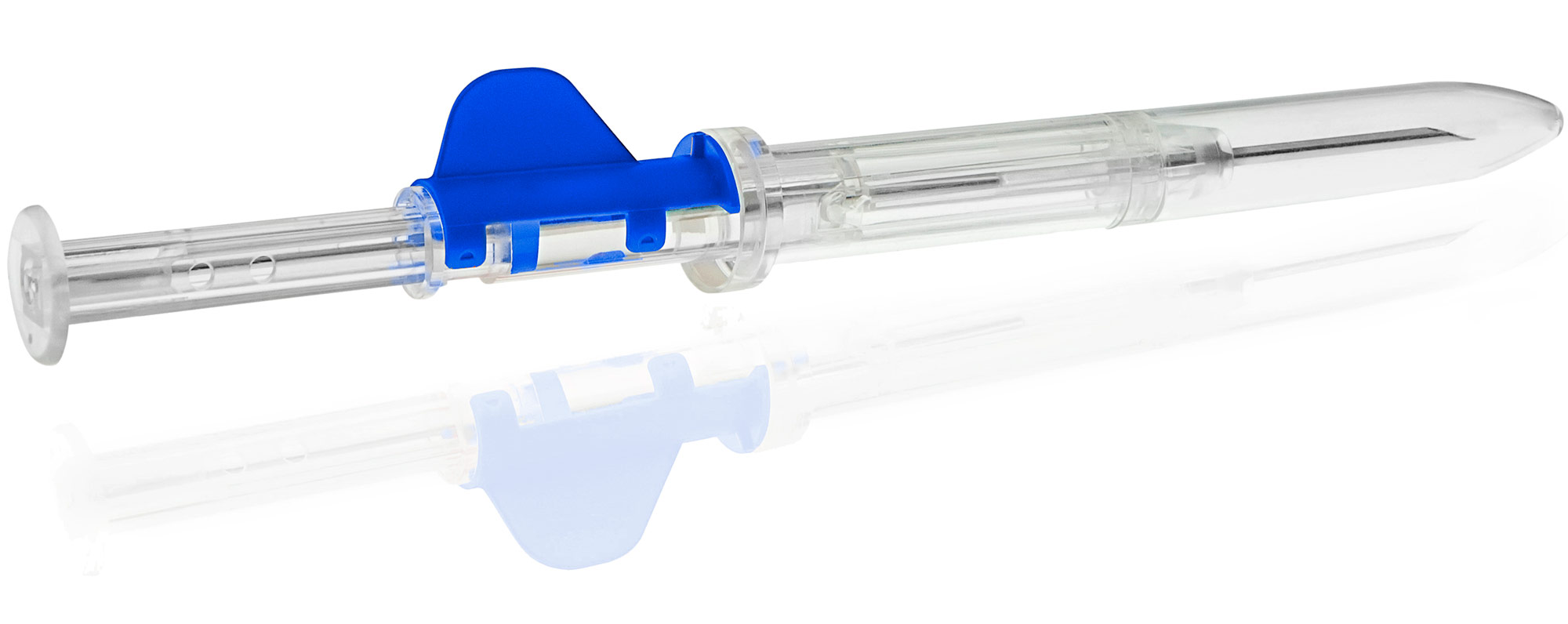
The active ingredient leuprorelin, like goserelin, is a synthetic peptide that can be used primarily in the treatment of hormone-dependent tumors, particularly prostate cancer. Administration of leuprorelin has indirect effects on testosterone levels in men, the lowering of which stops the growth of malignant cancerous tumors. Leuprorelin is administered in the form of a biodegradable implant that releases the active ingredient over a three-month period (11.25 mg dosage). Leuprorelin is the only implant formulation with a self-retracting needle, which makes administration much more convenient for patients.
Range of services
AMW only develops and licenses those products that have been manufactured in its own modern production facilities. Furthermore we manage all IP and regulatory aspects as legal owner.
In addition to the development of our proprietary pipeline, we keep a constant dialogue with innovative pharmaceutical and medtech companies regarding parenteral delivery systems to collaborate as CDMO partner (co-development or manufacturing).
Licensing
- OOut-licensing of existing and pipeline products for global submissions based on EU dossiers
- Contract development and contract manufacturing for drug delivery systems, e. g. implants, under GMP-conditions
- Sustainable life cylce management through continuous regulatory support and consistent process improvements
- Production of high-quality pharmaceuticals on state-of-the-art production lines
- Packaging products in line with market requirements thanks to high-performance packaging lines
Authorization
AMW experienced RA team provides efficient and reliable support to customers in planning, obtaining, and retaining marketing authorizations.
Services
- Advice on regulatory strategy
- Scientific advice procedures (in a meeting or in writing):
- Preparation (consultation, briefing document)
- Organization and implementation
- Preparation of product registration dossier (CTD modules 2-5; also complete module 1, if required)
- Product registration procedure: support including Replying to deficiency letters; if necessary, also preparation and complete execution of procedure
- Product lifecycle management:
- Preparation of variation documentation for submission
- Support during variation procedures, incl. Replying to deficiency letters; if necessary, also complete implementation of procedure
Approvals
So far, marketing authorizations have been obtained for Europe, Asia, Africa and Latinamerica for the following products developed by AMW via European DCPs or national procedures:
- Goserelin 3.6 mg prefilled syringe with implant
- Goserelin 10.8 mg prefilled syringe with implant
- Leuprorelin 11.25 mg prefilled syringe with implant
Your partner for product development and accessing new markets
To enable you to access international markets quickly and easily, we provide licensing models for high-quality drug patches and biodegradable implants developed by us in-house.
We partner with renowned pharmaceutical companies and offer our customers and international marketing and sales partners a complete package comprising licensing and support for our products, which we manufacture on site in Warngau. We grant licenses for the marketing of our products in all global markets. AMW is also the preferred partner for R&D collaborations.




